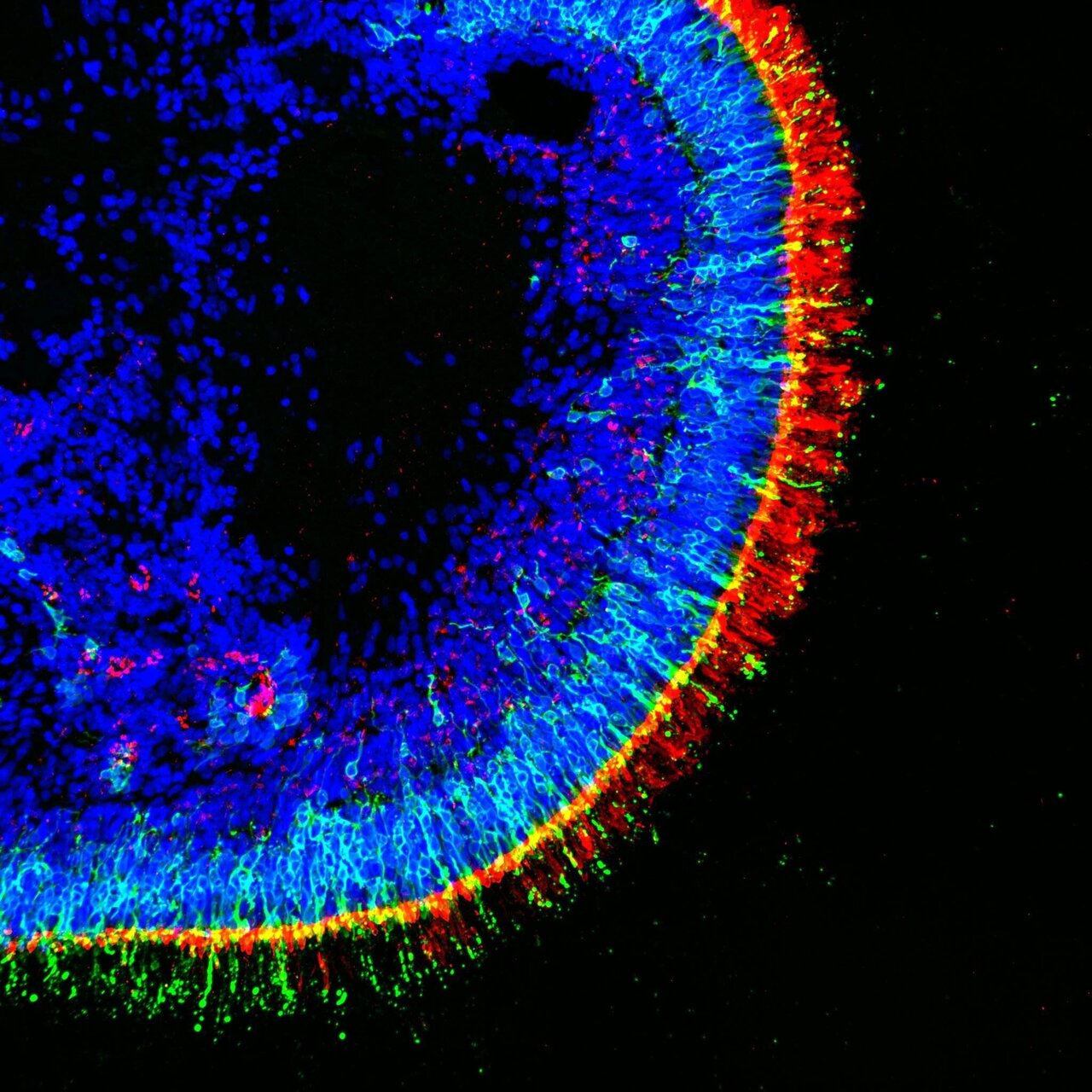
Picture, for a moment, a cluster of cells in the early stages of development. Guided by a carefully orchestrated sequence of signals, these cells arrange themselves into round structures that closely resemble your retina, the thin, layered tissue at the back of your eye that captures light. Retinal organoids are three-dimensional structures grown from stem cells in the lab, capturing key features of the human retina.
Rather than being flat, unsophisticated colonies, retinal organoids actually contain layered cells, rods, cones, and ganglion cells, much like the retinas that allow you to see the London Eye on a bright morning or the headlights on a rainy M25. The real draw here is the faithfulness with which these tiny imitations echo human biology. While they won’t let you see in the dark (yet), retinal organoids allow researchers to study diseases and treatments in a nuanced, dynamic way that animal models or flat cultures simply cannot mimic.
You will find, as you wade further into this topic, that organoids provide a unique vantage point, a sort of micro-lens, through which the mysteries of retinal health and disease come into focus.
How Retinal Organoids Are Generated
Creating a retinal organoid is a bit like cultivating a delicate garden, where the right conditions matter just as much as the seeds. Scientists start with pluripotent stem cells, often derived from your skin or blood. These cells possess remarkable plasticity and, when cajoled with the right biochemical cues, can transform into virtually any cell type, retinal cells included.
Your guide through this cellular maze is a precise cocktail of growth factors and nutrients. Over weeks (even months), these stem cells are gently nudged to divide and self-organise, forming structures that begin to resemble a developing human retina. If you were to peer through a laboratory microscope, you’d see layers forming, photoreceptors, bipolar cells, and more, each in its rightful place, echoing the complexity of your own eye.
It is never quite straightforward, mind. Variables like cell line choice, timing, and even the way cells are handled can alter the final result. But the transformative power here cannot be ignored: you end up with mini-organs that not only look the part, but actually respond to light and drugs much in the way your actual retina does.
Applications of Retinal Organoids in Research
You will find that retinal organoids have reignited curiosity in old and new corners of eye research. For one, there’s the direct observation they allow, rather than relying solely on animal models or post-mortem tissue, you can study living, developing human retina in a dish. Researchers use organoids to map the intricate choreography of retinal development, unpicking exactly how each cell type finds its place.
Gene editing, too, comes into play. You might have heard of scientists introducing or correcting genetic mutations in organoids to watch how disorders like retinitis pigmentosa or macular degeneration take hold. This hands-on, highly controlled method means you can test theories and probe mechanisms in real-time.
Collaborations between research teams and clinics are turning retinal organoids into a real bridge, where findings in the lab are fed back to clinical practice, and vice versa. There is a sort of quiet revolution happening in the way researchers ask questions about your vision and seek answers that could ripple outward to benefit many.
Retinal Organoids in Disease Modelling and Drug Testing
Few tools allow you to watch a disease unfold as it does in retinal organoids. If you are curious about how genetic disorders devastate eyesight, you can model these conditions, side by side with healthy controls. Changes in cell structure, responses to stress, or patterns of death and survival become visible.
You will find pharmaceutical researchers increasingly turning to organoids to test new drugs. There’s a certain relief in knowing that drug responses can be observed in genuine human tissue, without rushing straight to clinical trials. You can assess not only efficacy but also potential side effects, picking up subtle signs that animal models often miss. Reactions to treatments might reveal layers of complexity. Side effects might emerge that you cannot predict through conventional means, so drug pipelines begin to look a touch more reliable.
Personalised medicine is edging closer, too. In the case that a patient’s own cells are used, organoids let you test therapies tailor-made for them. The right treatment, for the right person, becomes a reality inch by inch.
Challenges and Limitations of Retinal Organoid Technology
Things are never as smooth in practice as they look under the microscope. You might think that organoids are perfect replicas, but the picture’s a bit patchy. Retinal organoids do miss some aspects of mature retina: fine-tuned vascular networks, certain supporting cell layers, even full functionality that matches real human eyes. If you were hoping for instant clinical fixes, patience is still essential.
You will run into reproducibility challenges. Variability between different stem cell lines, or even the hands sculpting the experiment, can alter outcomes. Ethical hurdles pop up, especially when sourcing stem cells and handling sensitive patient-derived material.
And there’s the issue of scale. Producing enough high-quality organoids for mass drug screening or transplantation remains a technical knot yet to be fully unravelled. Rigorous control, more consistent protocols, and ongoing dialogue with regulatory bodies, all these must blend together before retinal organoids can meet their full promise.
Future Perspectives and Potential Clinical Uses
The gleam in the eye of anyone passionate about retinal organoids lies in their future possibilities. You should know that support for clinical transplantation is growing. Can organoids, grown from a patient’s own cells, restore vision lost to disease? Trials are taking careful steps forward. Nothing rushed, but every tiny step is significant.
Biobanking, creating libraries of organoids from people with various eye conditions, could offer models for vast swathes of your population. It’s the groundwork for personalised medicine, where treatments are designed around a patient’s unique biology. In combination with gene therapy and new drug pipelines, the therapies of tomorrow seem just within your reach.
There’s talk of merging retinal organoids with microfluidic devices (‘organs on chips’), giving you even more fidelity in disease modelling. Artificial intelligence, too, is being harnessed to interpret the firehose of data produced as these micro-lenses are scrutinised in ever greater detail. You will find, in the years ahead, that the eye’s secrets are being teased apart in cleverer and less invasive ways than you have ever known before.
And Finally
Retinal organoids offer you a tantalising vantage point at the intersection of biology, technology, and human ambition. Though they are not a panacea, you will find that these micro-lenses change the way researchers approach questions once deemed unanswerable. The dance between the possible and the proven is ongoing, and if you keep your sights set on progress, the breakthroughs poised on the horizon might just catch you by surprise. Eyes open, science rarely blinks.